Pioneering neuro-metabolic therapies for high-need CNS disorders
- Two lead programs: an ASO for sporadic and genetic ALS patients targeting lipid biology, and an oral small molecule for schizophrenia targeting glutamate biology
- Lead programs on track for INDs this year, with clinical data in 2025
- Additional pipeline programs in Alzheimer’s disease, epilepsy, and depression
Pipeline
Program
Target / pathway
Indication
Modality
Preclinical
DC
IND-Enabling
Clinical
LTX-001
GLS1 / Excessive CNS glutamate
Schizophrenia, BD, MDD, ALS
Small molecule
LTX-002
SPTLC1 / Excessive CNS sphingolipids
ALS
ASO
LTX-007
SPT / Excessive CNS sphingolipids
Alzheimer’s Disease, rare sphingolipidoses, obesity
Small molecule
LTX-003, LTX-004
Multiple
Alzheimer’s Disease
Pipeline
Program: LTX-001
Modality: Small molecule
Target: GLS1 / Excessive CNS glutamate
Indication: Schizophrenia, BD, MDD, ALS
Pre-clinical
DC
IND-enabling
Clinical
Program: LTX-002
Modality: ASO
Target: SPTLC1 / Excessive CNS sphingolipids
Indication: ALS
Pre-clinical
DC
IND-enabling
Clinical
Program: LTX-007
Modality: Small molecule
Target: SPT / Excessive CNS sphingolipids
Indication: Alzheimer’s Disease, rare sphingolipidoses, obesity
Pre-clinical
DC
IND-enabling
Clinical
Program: LTX-003, LTX-004
Modality: ASO
Target: Multiple
Indication: Alzheimer’s Disease
Pre-clinical
DC
IND-enabling
Clinical
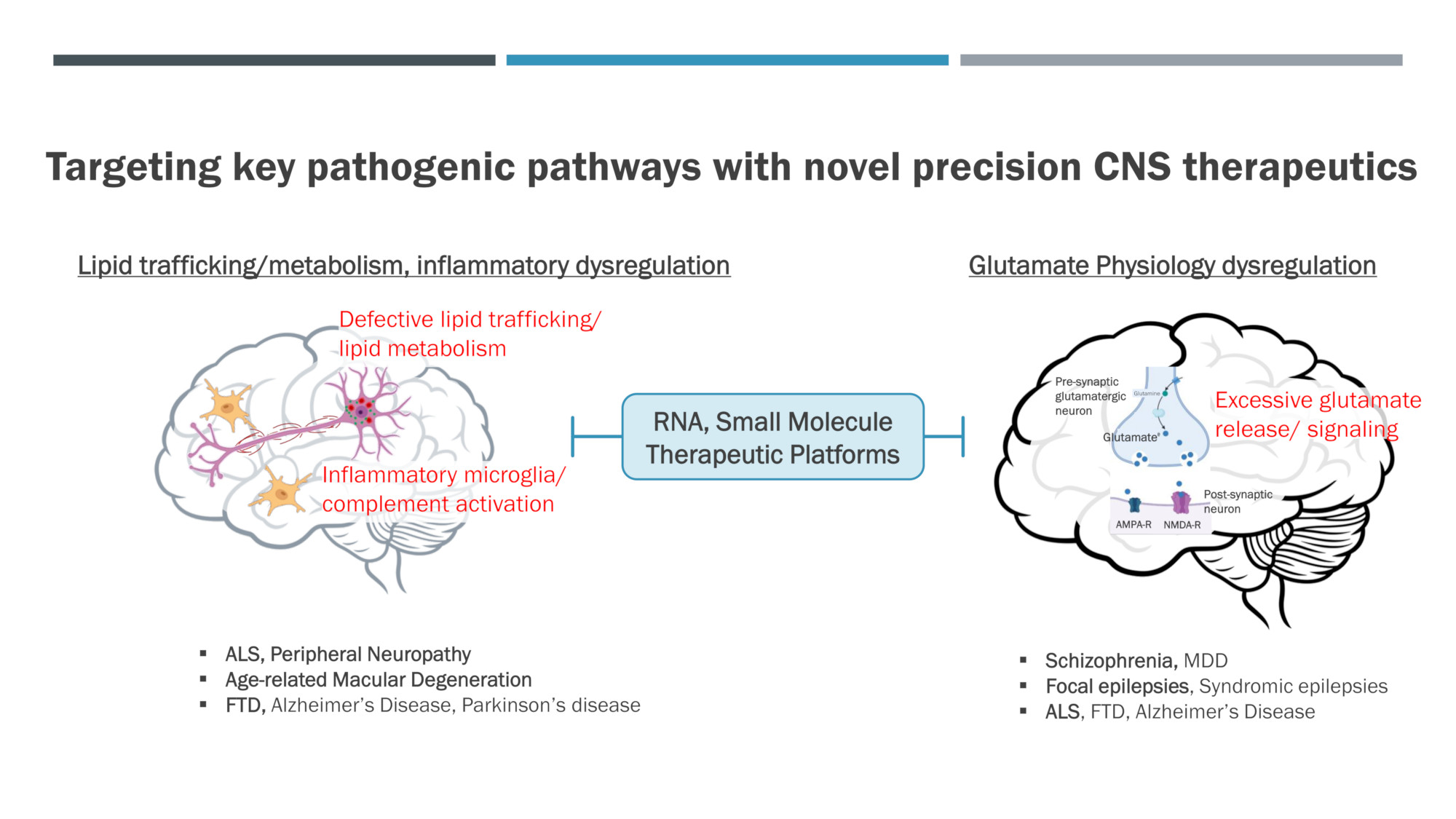
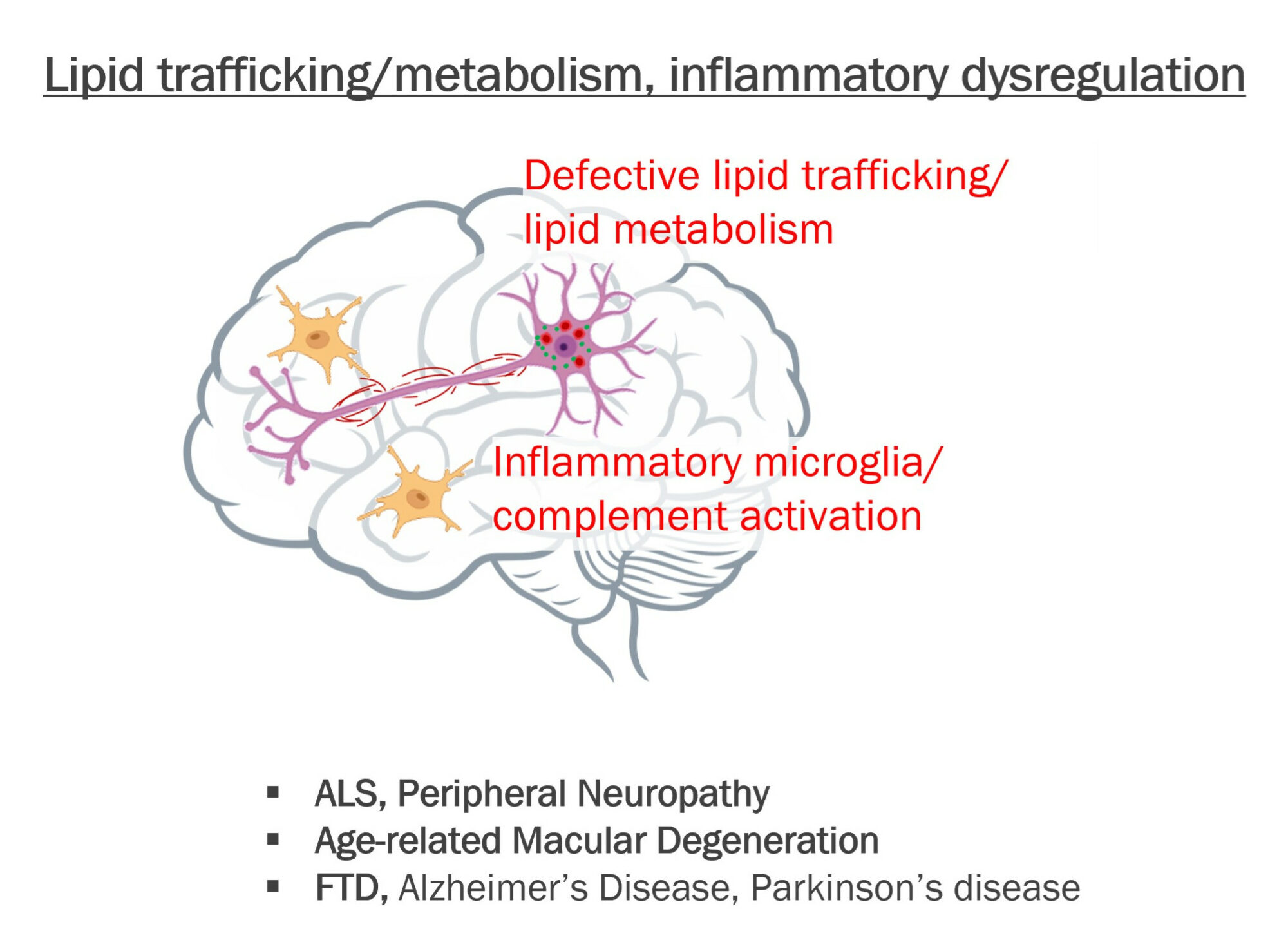
Developing novel precision CNS therapeutics that target key pathogenic pathways