Pioneering precision medicines for high-need CNS disorders
- Two lead programs: an ASO for sporadic and genetic ALS patients targeting lipid biology, and an oral small molecule for schizophrenia targeting glutamate biology
- Lead programs on track for INDs this year, with clinical data in 2025
- Additional pipeline programs in Alzheimer’s disease, epilepsy, and depression
Pipeline
Program
Target
Indication
Modality
Leads
Preclinical
DC
IND-Enabling
Clinical
LTX-002
SPTLC1 /
Lipid metabolism
LTX-001
GLS1 /
Glutamate presynaptic
Schizophrenia, MDD, ALS
Small molecule
LTX-003
Alzheimer’s Disease, FTD, AMD
ASO
LTX-004
LTX-005
Glutamate NMDAR
Refractory Epilepsy, MDD/TRD
ASO
Pipeline
Program: LTX-002
Modality: ASO
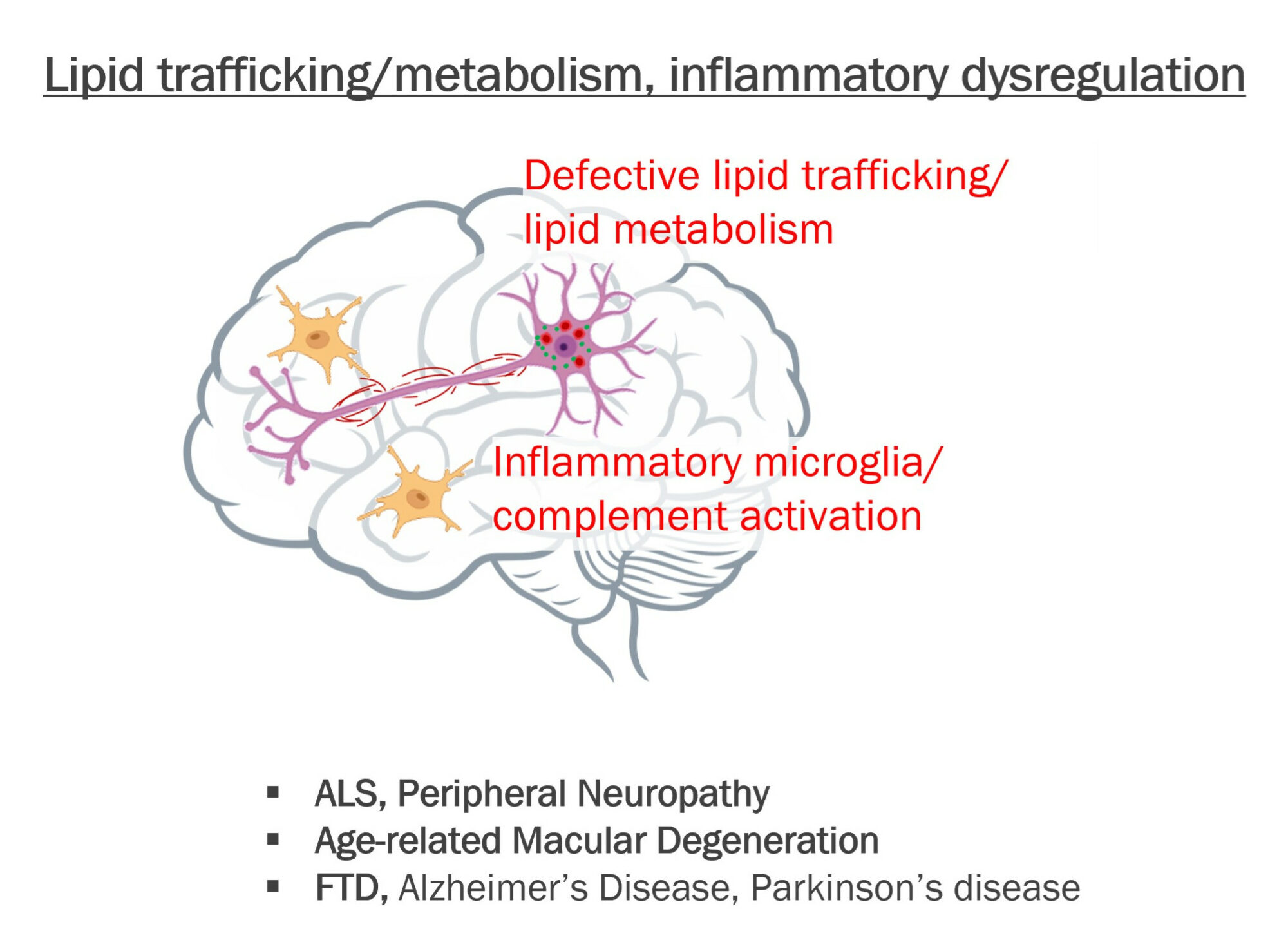
Target: SPTLC1 / Lipid metabolism
Indication: ALS, Peripheral Neuropathy, Alzheimer’s Disease
Leads
Pre-clinical
DC
IND-enabling
Clinical
Program: LTX-001
Modality: Small molecule
Target: GLS1 / Glutamate presynaptic
Indication: Schizophrenia, MDD,
ALS
Leads
Pre-clinical
DC
IND-enabling
Clinical
Program: LTX-003
Modality: ASO
Target: Complement
Indication: Alzheimer’s Disease, FTD, AMD
Leads
Pre-clinical
DC
IND-enabling
Clinical
Program: LTX-004
Modality: ASO
Target: Lipid trafficking
Indication: Alzheimer’s Disease, Dementia with Lewy bodies
Leads
Pre-clinical
DC
IND-enabling
Clinical
Program: LTX-005
Modality: ASO
Leads
Pre-clinical
DC
IND-enabling
Clinical
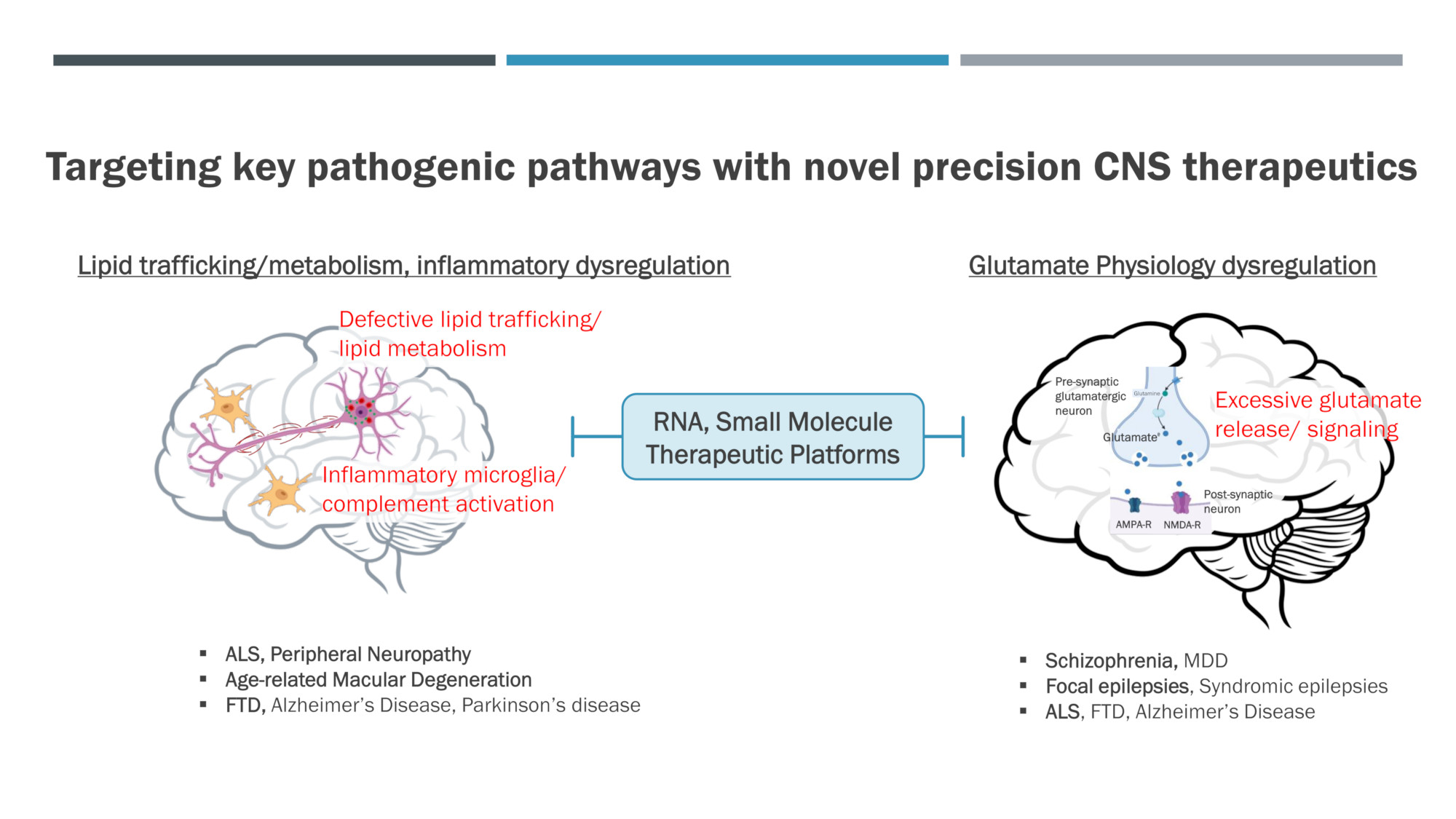
Developing novel precision CNS therapeutics that target key pathogenic pathways